Rabeprazole & Domperidone DSR Capsule
77 INR/Box
Product Details:
- Drug Type General Medicines
- Physical Form Capsules
- Dosage 30 mg
- Suitable For Adults
- Storage Instructions Cool and Dry Place
- Click to view more
X
Rabeprazole & Domperidone DSR Capsule Price And Quantity
- 300 Box
- 77 INR/Box
Rabeprazole & Domperidone DSR Capsule Product Specifications
- 30 mg
- Adults
- Cool and Dry Place
- Capsules
- General Medicines
Rabeprazole & Domperidone DSR Capsule Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 300 Box Per Month
- 1 Months
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Western Europe Asia Eastern Europe Middle East Central America South America North America Australia Africa
- All India
Product Description
Rapebrazole is widely utilized to prevent and heal gastrointestinal (stomach and guts) problems, which is caused due to overmuch stomach acid. Rapebrazole is a proton pump inhibitor (PPI), sometimes identified as an ulcer healing drug. This is also very beneficial for inhibiting secretion and gastric from the stomach as well as to treat various acid related gastrointestinal conditions. It heals stomach and intestinal ulcers and manages gastro-oesophageal reflux disease (GORD a condition in which acid rises up from the stomach into the oesophagus [gullet] with symptoms of heartburn, acid regurgitation and pain on swallowing).Domperidone is a dopamine antagonist with anti-emetic properties domperidone does not readily cross the blood-brain barrier. Its anti-emetic effect may be due to a combination of peripheral (gastrokinetic) effects and antagonism of dopamine receptors in the chemoreceptor trigger zone, which lies outside the blood-brain barrier in the area postrema. Studies in man have shown oral domperidone to increase lower esophaegeal pressure, improve antroduodenal motility and accelerate gastric emptying. There is no effect on gastric secretion.
Indications and Usage:
Gerd, Heart Burn & Hyperacidity, Reflux Oesophagitis, Regurgitation & Flatulance,Gastric & Peptic Ulcer.
Mechanism of Action:
Rabeprazole is a partially reversible inhibitor of H+K+ ATPase which is activated in the acidic lumen ofthe gastric parietal cells. The canalicular membrane of the gastric parietal cells contains the protonpump - H+K+ ATPase enzyme . It exchanges H+ ions for K+ ions using energy generated by thebreakdown of ATP to ADP. This enzyme represents the final step for acid production in the stomach. Itdissociates more quickly and completely from H+/K+ ATPase than omeprazole.
| Domperidone increaseslower esophageal sphinctertone and enhances upper GImotility, thereby preventingreflux of gastric contents into esophagus. |
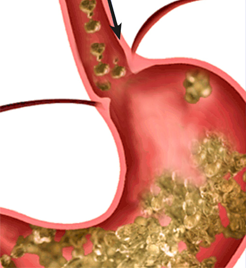 |
Rabeprazole inhibits theH+-K+-ATPase enzyme and thereby decreases gastricacid secretion. |
Drug Interactions:
Rabeprazole :
pH dependent interactions with digoxin and ketoconazole.
Domperidone :
a). Concomitant administration of anticholinergic drugs may decrease the effect of domperidone.
b). Azole antifungals / macrolide antibiotic, increases plasma levels of domperidone.
Rationale for Combination:
Rabeprazole being the fastest acting PPI gives a quick relief from the hyper acid secretory conditions. Asignificant percentage of GERD patients have delayed gastric emptying10 and hypotensive esophagealsphincter.
Domperidone, a prokinetic and anti-emetic improves the LES tone, increases the gastric motility andthus helps in gastric emptying. In GERD patients not responding to Rabeprazole alone, combination ofRabeprazole and Domperidone may be effective.
Pharmacokinetics:
Rabeprazole:
Absorption:Rabeprazole sodium is acid labile and formulated as an enteric coatedtablet. It is rapidly, dose dependently absorbed after oral administration. Food doesnot affect the absorption or the peak plasma concentration but delays the time to peakplasma concentration (tmax) by about 1.5 hours. Bioavailability is not influenced by theco-ingestion of either food or antacids.
Distribution:Rabeprazole is 97% bound to human plasma proteins.
Metabolism:It undergoes extensive metabolism via nonenzyme metabolism to form major inactivemetabolites (a thioether carboxylic acid metabolite and its glucuronide) and also minor metabolism byCYP2C19 and CYP 3 A4 to desmethyl and sulfone metabolites. Rabeprazole does not accumulatesignificantly during repeated administration.
| tmax | 3.5 hours |
| t1/2 (half life) | 0.7 - 1.5 hours |
| Plasma protein binding | 97% |
| Oral bioavailability | ~52% |
| Excretion | ~90% in urine (metabolites) ~10% in faeces (unchanged) |
Excretion:Approximately 90% of the drug was eliminated in urine, primarily as thioether carboxylicacid; its glucoronise and mercapturic acid metabolites. The remainder of the dose is recovered in thefaeces.
Domperidone:
Absorption:Domperidone available in PARIT-D capsules is in the form of controlledreleasepellets, facilitating the use of PARIT-D as once daily dosage. In vitro dissolution testusing 0.1 N HCl at 37 ± 5°C demonstrated a release of 15 - 40%, 30 - 60% and 55 - 85% ofdomperidone from PARIT-D at 1, 4 and 8 hours respectively. The released domperidone israpidly absorbed following oral administration.26 Systemic bioavailability of oral domperidone is13 - 17%, probably the result of hepatic first-pass and gut wall metabolism. The half-life is approximately 7.0hours. Oral bioavailability is decreased by prior administration of cimetidine or sodium bicarbonate.
Distribution:Oral domperidone does not appear to accumulate or to induce its own metabolism; apeak plasma level after 90 minutes of 21 ng/mL after two weeks oral administration of 30 mg per daywas almost the same as that of 18 ng/mL after the first dose. Domperidone is 91 - 93% bound to plasmaproteins.26 It does not readily cross the blood brain barrier and therefore is not expected to have centraleffects.22 However, according to animal studies, very low amounts cross the placental barrier and it isexcreted in the breast milk.
Metabolism:Oral domperidone does not appear to accumulate or induce its own metabolism. Itundergoes rapid and extensive hepatic metabolism by hydroxylation and N-dealkylation. In vitrometabolism experiments with diagnostic inhibitors revealed that CYP3A4 is a major form of cytochromeP-450 involved in the N-dealkylation of domperidone, whereas CYP3A4, CYP1A2 and CYP2E1 areinvolved in domperidone aromatic hydroxylation.
Elimination:Urinary and faecal excretion amounts to 31 and 66%, respectively, of the oral dose. Theproportion of the medicine excreted unchanged is small (10% of faecal excretion and approximately 1%of urinary excretion). The plasma half-life after a single oral dose is 7-9 hours in healthy subjects but isprolonged in patients with severe renal insufficiency.
Side Effects:
Generally well tolerated. Most common side effects are somnolence, dizziness, dry mouth and blurring of vision
Storage:
Store at room temperature, 15-30° C (59-86°F). Keep away from moisture.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email