
Terbinafine Tablet
71 INR/Box
Product Details:
- Purity 99%
- Formulations Type General Drugs
- Formulations Form Tablets
- Gender/Age Group Adult
- Storage Instructions Dry Place
- Click to view more
X
Terbinafine Tablet Price And Quantity
- 300 Box
- 71 INR/Box
Terbinafine Tablet Product Specifications
- 99%
- General Drugs
- Tablets
- Adult
- Dry Place
Terbinafine Tablet Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 300 Box Per Month
- 1 Months
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Western Europe Asia North America Eastern Europe Central America Middle East South America Australia Africa
- All India
Product Description
Terbinafine (HCl) is antifungal agent, it is a synthetic allylamine and available as oral and topical formulation. Oral terbinafine is highly effective for treating onychomycosis due to its fungicidal activity and ability to concentrate within the nails. It was developed by Novartis. It is highly lipophilic in nature and tends to accumulate in skin, nails, and fatty tissues. The U.S. Food and Drug Administration approved the first generic versions of terbinafine hydrochloride tablets. On September 28, 2007, FDA approved the use of terbinafine hydrochloride use in children of age 4 and above.They are potent fungicidal agents as it inhibits ergosterol synthesis by inhibiting the fungal squalene monooxygenase (squalene 2,3-epoxidase), an enzyme that is part of the fungal cell wall synthesis pathway, thereby removing the fungal infection from the root level. It is useful in various fungal disorders such as Athelets foot, Dermatophyte infections of the nails, Hair and nail infection, scalp Ringworm, Tinea corporis, Tinea cruris, Tinea pedis.
Indication and Usage:
 |
 |
 |
 |
Tinea cruris, Tinea pedis.
Mechanism of Action:
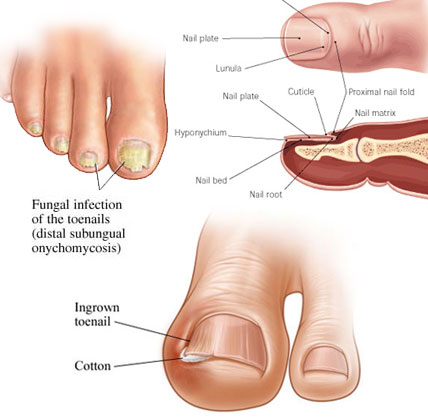 Terbinafine acts by inhibiting squalene monooxygenase, thus blocking the biosynthesis of ergosterol, an essential component of fungal cell membranes. This inhibition also results in an accumulation of squalene, which is a substrate catalyzed to 2,3-oxydo squalene by squalene monooxygenase. The resultant high concentration of squalene and decreased amount of ergosterol are both thought to contribute to terbinafine's antifungal activity.
Terbinafine acts by inhibiting squalene monooxygenase, thus blocking the biosynthesis of ergosterol, an essential component of fungal cell membranes. This inhibition also results in an accumulation of squalene, which is a substrate catalyzed to 2,3-oxydo squalene by squalene monooxygenase. The resultant high concentration of squalene and decreased amount of ergosterol are both thought to contribute to terbinafine's antifungal activity.Drug Interactions:
Decreased terbinafine concentration with rifampicin; increased terbinafine concentration with cimetidine.
Pharmacology:
Pharmacokinetic:
Absorption: Absorbed well from the GI tract with 40% bioavailability (oral), minimal absorption (topical); peak plasma concentrations after 2 hours (oral).
Distribution:Distributed into stratum corneum of the skin, nail plate, hair (concentrations higher than plasma) and breastmilk. Protein-binding: Extensive.
Metabolism: Hepatic; converted to inactive metabolites.
Excretion: Via urine; 17-36 hours (plasma elimination half-life); up to 400 hours (terminal elimination half-life) in prolonged therapy.
Adverse Effects:
Anorexia, nausea, abdominal pain, taste disturbances, diarrhoea, rash, urticaria.
Contraindications:
â— Caution when used during pregnancy
â— Safety and efficacy not established in children younger than 4 years of age.
Warning and Precaution:
Terbinafine Hydrochloride tablets are not recommended for patients with chronic or active liver disease. Before prescribing Terbinafine Hydrochloride Tablets, pre-existing liver disease should be assessed. Hepatotoxicity may occur in patients with and without pre-existing liver disease. Pretreatment serum transaminase (ALT and AST) tests are advised for all patients before taking Terbinafine Hydrochloride Tablets.
Patients prescribed Terbinafine Hydrochloride Tablets should be report immediately to their physician of any symptoms of persistent nausea, anorexia, fatigue, vomiting, right upper abdominal pain or jaundice, dark urine or pale stools. Patients with these symptoms should discontinue taking oral Terbinafine, and the patient's liver function should be immediately evaluated.
Conclusion:
From the above discussion we can conclude that terbinafine can control symptoms of various fungal diseases, and aids in healthy life.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email







